
The role of a Clinical Research Coordinator (CRC) is pivotal in the healthcare and pharmaceutical industries, serving as the vital link between clinical trials and patient care. CRCs are responsible for managing and overseeing clinical research studies, ensuring compliance with regulatory standards while collecting and analyzing data. In today’s job market, the demand for skilled CRCs is surging, driven by the growing need for innovative treatments and therapies. This article will provide a comprehensive guide to crafting an effective resume for aspiring Clinical Research Coordinators, highlighting key skills and strategies to stand out in this competitive field.
- Clinical Research Coordinator resume examples
- How to format a Clinical Research Coordinator resume
- How to write your Clinical Research Coordinator resume experience
- How to list your hard skills and soft skills on your resume
- How to list your certifications and education on your resume
- How to write your Clinical Research Coordinator resume summary or objective
- Additional sections for a Clinical Research Coordinator resume
- Key takeaways for writing a professional Clinical Research Coordinator resume
- Frequently Asked Questions
Clinical Research Coordinator resume examples
Clinical Research Coordinator resume examples serve as valuable resources for job seekers aiming to excel in this specialized field. By analyzing these examples, candidates can gain insights into effective resume formatting, essential skills, and impactful language that highlight their qualifications. Understanding what makes a strong resume can significantly enhance a candidate’s chances of standing out in a competitive job market, ultimately leading to successful career opportunities.
Clinical Research Coordinator Resume

Why This Resume Works
This resume effectively positions the candidate for a Clinical Research Coordinator role by highlighting 12 years of relevant experience, underscored by key skills like Clinical Trial Management and Regulatory Compliance. Its clear format enhances readability, showcasing achievements that align with industry standards. The use of specific keywords ensures ATS compatibility, making it easier for the resume to pass through automated screenings. Overall, this strategic presentation emphasizes both expertise and accomplishments critical to success in clinical research coordination.
Clinical Trials Manager Resume

Why This Resume Works
This resume effectively positions the candidate for a Clinical Trials Manager role by highlighting essential skills such as regulatory compliance and project management, which are crucial in this field. With approximately 7 years of relevant experience, including roles like Clinical Research Associate and Coordinator, it demonstrates a strong career trajectory. The format is clear and organized, enhancing readability for hiring managers and ATS systems alike. Additionally, strategic presentation of achievements showcases the candidate’s impact on study efficiency and stakeholder engagement, aligning perfectly with industry demands.
Research Study Lead Resume
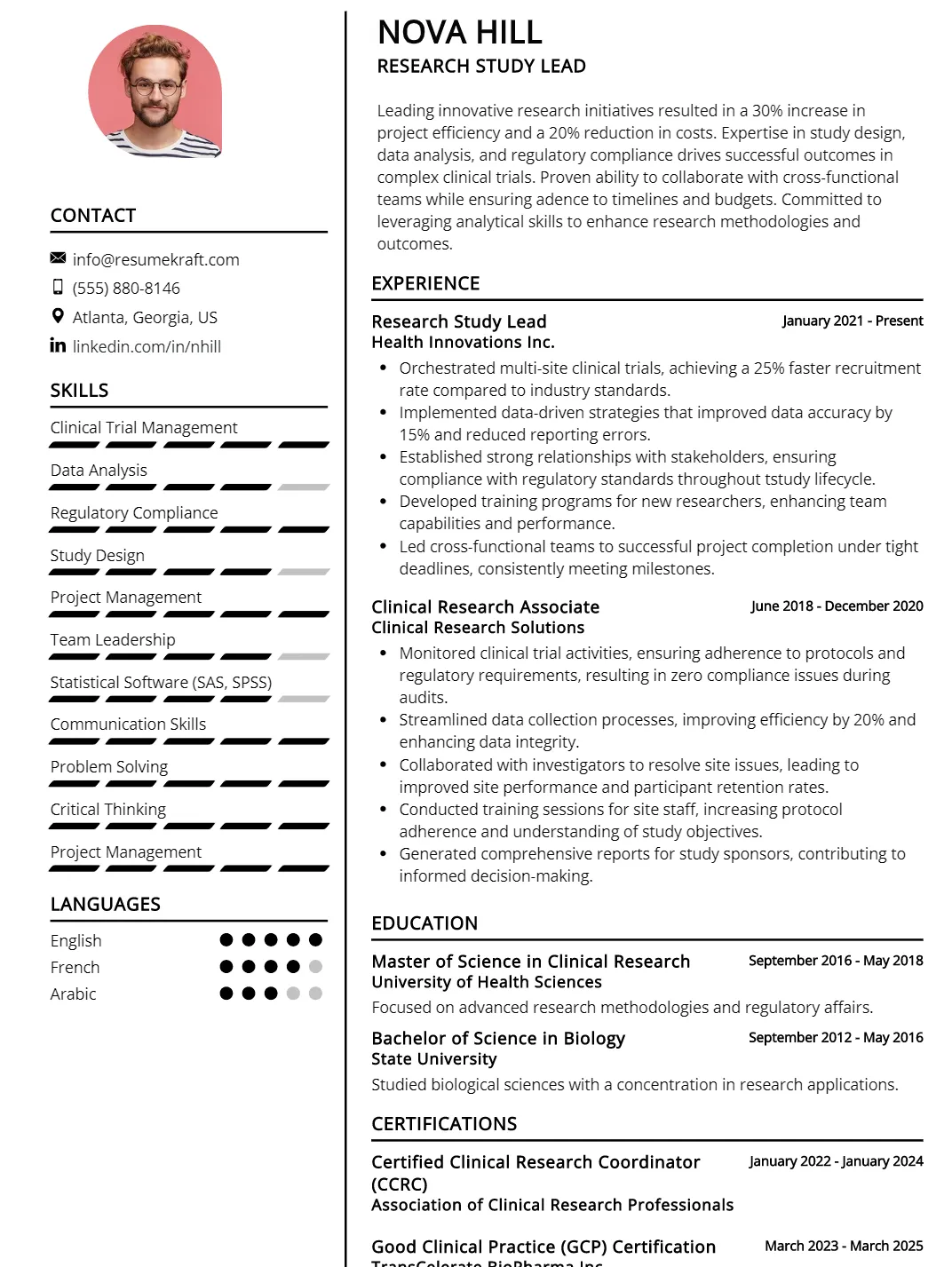
Why This Resume Works
This resume is effective for a Research Study Lead position due to its targeted skill set, including clinical trial management and regulatory compliance, which directly align with job requirements. The structured format highlights relevant experience as a Research Study Lead and Clinical Research Associate, showcasing approximately six years in the field. It ensures ATS compatibility by incorporating industry-specific keywords. Additionally, the strategic presentation of quantifiable achievements emphasizes proficiency in study design and project management, making it stand out to hiring professionals seeking expertise in research.
Clinical Research Associate Resume

Why This Resume Works
This resume effectively highlights the candidate’s five years of relevant experience as a Clinical Research Associate and Assistant, showcasing essential skills like clinical trial management and regulatory compliance. The structured format emphasizes key competencies, making it easy for hiring managers to assess qualifications quickly. By incorporating industry-specific keywords, the resume enhances ATS compatibility, ensuring visibility during initial screenings. Additionally, the strategic presentation of achievements related to patient recruitment and protocol development underscores the candidate’s impact in previous roles, reinforcing their suitability for the position.
Clinical Research Coordinator with Oncology Specialization Resume

Why This Resume Works
This resume is effective for a Clinical Research Coordinator with Oncology Specialization due to its emphasis on relevant skills like Clinical Trial Management and Protocol Development, directly aligning with industry needs. With 13 years of progressive experience in clinical research roles, it showcases a robust background that enhances credibility. The clear format and structured layout facilitate easy navigation, while the use of industry-specific keywords ensures ATS compatibility.
Junior Research Coordinator Resume
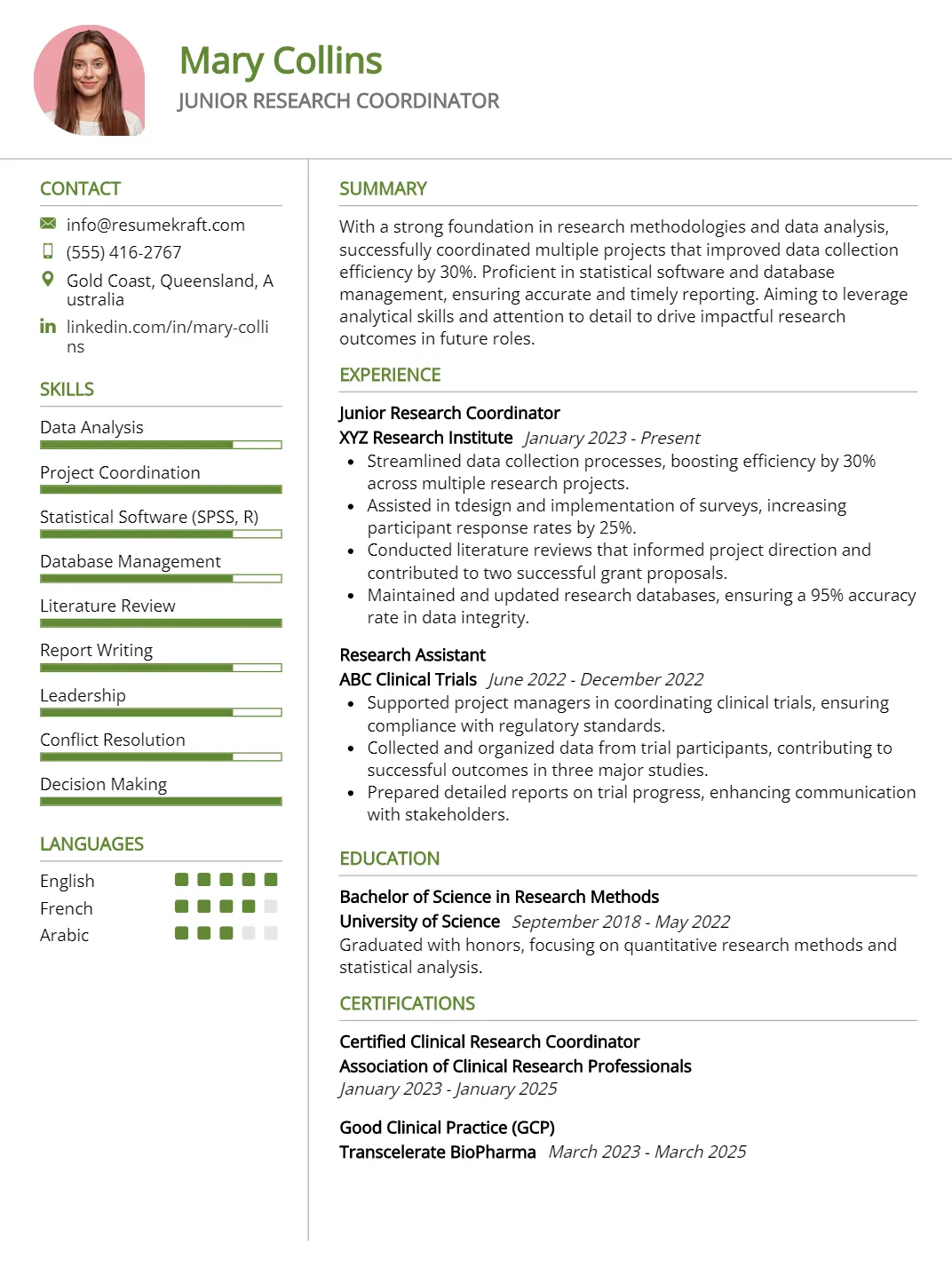
Why This Resume Works
This resume effectively highlights the candidate’s relevant skills and experience for a Junior Research Coordinator position by showcasing expertise in data analysis, project coordination, and statistical software like SPSS and R. Its clear structure emphasizes important qualifications, making it easy to read for hiring managers. Additionally, the use of industry-specific keywords ensures ATS compatibility, increasing visibility in applicant tracking systems. Strategic presentation of achievements related to database management and literature reviews further underscores their suitability for research-driven roles within this field.
Senior Research Study Manager Resume

Why This Resume Works
This resume effectively highlights the candidate’s extensive experience as a Senior Research Study Manager, showcasing relevant skills in clinical trial management and regulatory compliance. The structured format clearly delineates key competencies and achievements, making it easy for hiring managers to assess qualifications quickly. Additionally, its strategic use of industry-specific keywords enhances ATS compatibility, ensuring visibility in automated screenings. By emphasizing quantifiable achievements in project management and statistical analysis, the resume aligns perfectly with the expectations for this senior-level position, demonstrating readiness to excel.
Biomedical Project Coordinator Resume

Why This Resume Works
This resume effectively positions the candidate for a Biomedical Project Coordinator role by highlighting 13 years of progressive experience in project management, clinical trials coordination, and regulatory compliance. The clear format emphasizes key skills relevant to the position while ensuring ATS compatibility through targeted keywords. Additionally, strategic presentation of achievements showcases proven stakeholder engagement and data analysis capabilities, demonstrating the candidate’s ability to drive successful outcomes in biomedical projects. Overall, it aligns perfectly with industry expectations and demands.
Entry-Level Clinical Support Coordinator Resume

Why This Resume Works
This resume effectively highlights relevant skills such as Patient Coordination and Electronic Health Records (EHR), crucial for an Entry-Level Clinical Support Coordinator. The structured format emphasizes key experiences, like a Clinical Support Coordinator role, ensuring clarity and easy navigation for hiring managers. It is ATS-compatible with industry-specific keywords that enhance visibility. Additionally, the strategic presentation of accomplishments in appointment scheduling and customer service demonstrates tangible contributions to patient care, making it a strong contender for this position.
Clinical Research Coordinator (Transitional Resume) Resume

Why This Resume Works
This resume effectively highlights the candidate’s extensive 14 years of experience in clinical research, showcasing key skills like Clinical Trial Management and Regulatory Compliance that are critical for a Clinical Research Coordinator (Transitional) role. The structured format emphasizes achievements relevant to patient recruitment and team coordination, enhancing its appeal. Additionally, it incorporates industry-specific keywords to ensure ATS compatibility, making it easily discoverable. Overall, this tailored presentation aligns closely with the job requirements and demonstrates a strong fit for the position.
Senior Clinical Research Scientist Resume

Why This Resume Works
This resume effectively positions the candidate for a Senior Clinical Research Scientist role by highlighting essential skills such as Clinical Trial Design, Data Analysis, and Regulatory Compliance. The structured format ensures clarity and easy navigation, critical for busy hiring managers. Its ATS-compatible design incorporates relevant keywords, enhancing visibility in applicant tracking systems. Additionally, the strategic presentation of achievements demonstrates the candidate’s impact on previous projects, showcasing their ability to lead successful research initiatives in line with industry standards and expectations.
Senior Clinical Research Coordinator (Neuroscience Specialization) Resume

Why This Resume Works
This resume effectively highlights the candidate’s eight years of relevant experience, showcasing their expertise in clinical trial management and neuropharmacology—crucial for a Senior Clinical Research Coordinator in neuroscience. Its structured format presents key skills clearly, enhancing readability for hiring managers and ensuring ATS compatibility. By emphasizing achievements in regulatory compliance and patient recruitment strategies, the resume strategically aligns with industry demands, making it stand out as an ideal fit for this specialized role in clinical research.
Senior Clinical Project Manager Resume

Why This Resume Works
This resume effectively highlights the candidate’s extensive experience and key skills relevant to a Senior Clinical Project Manager role, showcasing seven years of progressive responsibility in clinical project management. The structured format emphasizes core competencies like project management and regulatory compliance, making it easy for hiring managers to identify qualifications. Its alignment with ATS requirements ensures visibility during initial screenings. Additionally, the strategic presentation of achievements demonstrates measurable impacts in previous positions, reinforcing the candidate’s capability to drive successful clinical trials within cross-functional teams.
Clinical Research Operations Specialist Resume

Why This Resume Works
This resume effectively showcases the candidate’s qualifications for a Clinical Research Operations Specialist position by highlighting relevant skills such as Clinical Trial Management and Regulatory Compliance. With approximately five years of experience, including roles as a Clinical Research Operations Specialist and Clinical Trial Assistant, the candidate demonstrates a strong background in project coordination and risk management. The clear, structured format enhances readability while ensuring ATS compatibility through targeted keywords.
Principal Clinical Research Coordinator Resume

Why This Resume Works
This resume effectively positions the candidate for the Principal Clinical Research Coordinator role by highlighting key skills such as Clinical Trial Management and Regulatory Compliance, directly aligning with industry demands. With nearly nine years of progressive experience, it showcases a solid career trajectory in clinical research. The structured format enhances readability, catering to both hiring managers and ATS compatibility. Additionally, strategic presentation of achievements in patient recruitment and budget management underscores the candidate’s impact in previous roles, making them an appealing choice for this position.
Clinical Research Coordinator Resume

Why This Resume Works
This resume effectively highlights the candidate’s extensive experience as a Senior Clinical Research Coordinator, showcasing 13 years in the field. The inclusion of key skills like Project Management and Regulatory Compliance aligns perfectly with the requirements for a Clinical Research Coordinator role. Its clear, structured format enhances readability while emphasizing relevant achievements, such as successful patient recruitment metrics. Additionally, the use of industry-specific keywords ensures ATS compatibility, making it easier for hiring managers to identify qualified candidates in a competitive job market.
Clinical Research Data Manager Resume

Why This Resume Works
This resume effectively showcases the candidate’s extensive experience and relevant skills for a Clinical Research Data Manager position. Highlighting key competencies like data management, regulatory compliance, and statistical analysis aligns perfectly with industry requirements. The structured format enhances readability, ensuring clarity for both hiring managers and ATS software. Strategic presentation of achievements in previous roles emphasizes successful project completions and contributions to clinical trials, making the candidate stand out as a well-qualified applicant ready to excel in data-driven environments.
Regulatory Affairs Specialist in Clinical Research Resume

Why This Resume Works
This resume effectively highlights the candidate’s expertise in regulatory compliance and clinical trial management, directly aligning with the requirements of a Regulatory Affairs Specialist in Clinical Research. The structured format emphasizes key skills and relevant experience, enhancing readability for hiring managers. By incorporating industry-specific terminology, it ensures compatibility with Applicant Tracking Systems (ATS). Additionally, the strategic presentation of achievements showcases successful submission preparations and adherence to FDA regulations, demonstrating the candidate’s capability to excel in this role.
Clinical Research Site Director Resume

Why This Resume Works
This resume effectively highlights the candidate’s extensive experience as a Clinical Research Site Director and Clinical Research Coordinator, emphasizing key skills like Clinical Trial Management and Regulatory Compliance that are crucial for the role. The clear format showcases achievements in Patient Recruitment and Budget Management, aligning with industry expectations. Its structured layout enhances readability for hiring managers and ensures ATS compatibility by incorporating relevant keywords specific to clinical research.
Patient Recruitment Coordinator for Clinical Trials Resume

Why This Resume Works
This resume effectively showcases the candidate’s extensive 13 years of relevant experience, particularly in patient recruitment and clinical trial management, making it highly suitable for a Patient Recruitment Coordinator role. The clear structure highlights key skills such as regulatory compliance and community outreach, essential for this position. Its ATS-friendly format ensures that crucial keywords are easily identified. Additionally, the strategic presentation of achievements demonstrates a proven track record in enhancing recruitment strategies and fostering patient engagement, directly aligning with industry demands.
Clinical Quality Assurance Auditor Resume

Why This Resume Works
This resume effectively highlights the candidate’s relevant skills and experience for a Clinical Quality Assurance Auditor position, showcasing expertise in clinical audit management and GCP/GLP compliance. The structured format emphasizes key competencies like data analysis and risk assessment, making it easy for hiring managers to identify qualifications. Additionally, the use of industry-specific keywords enhances ATS compatibility. Strategic presentation of achievements related to quality improvement initiatives further demonstrates the candidate’s impact in previous roles, setting them apart in this competitive field.
Global Clinical Research Program Manager Resume
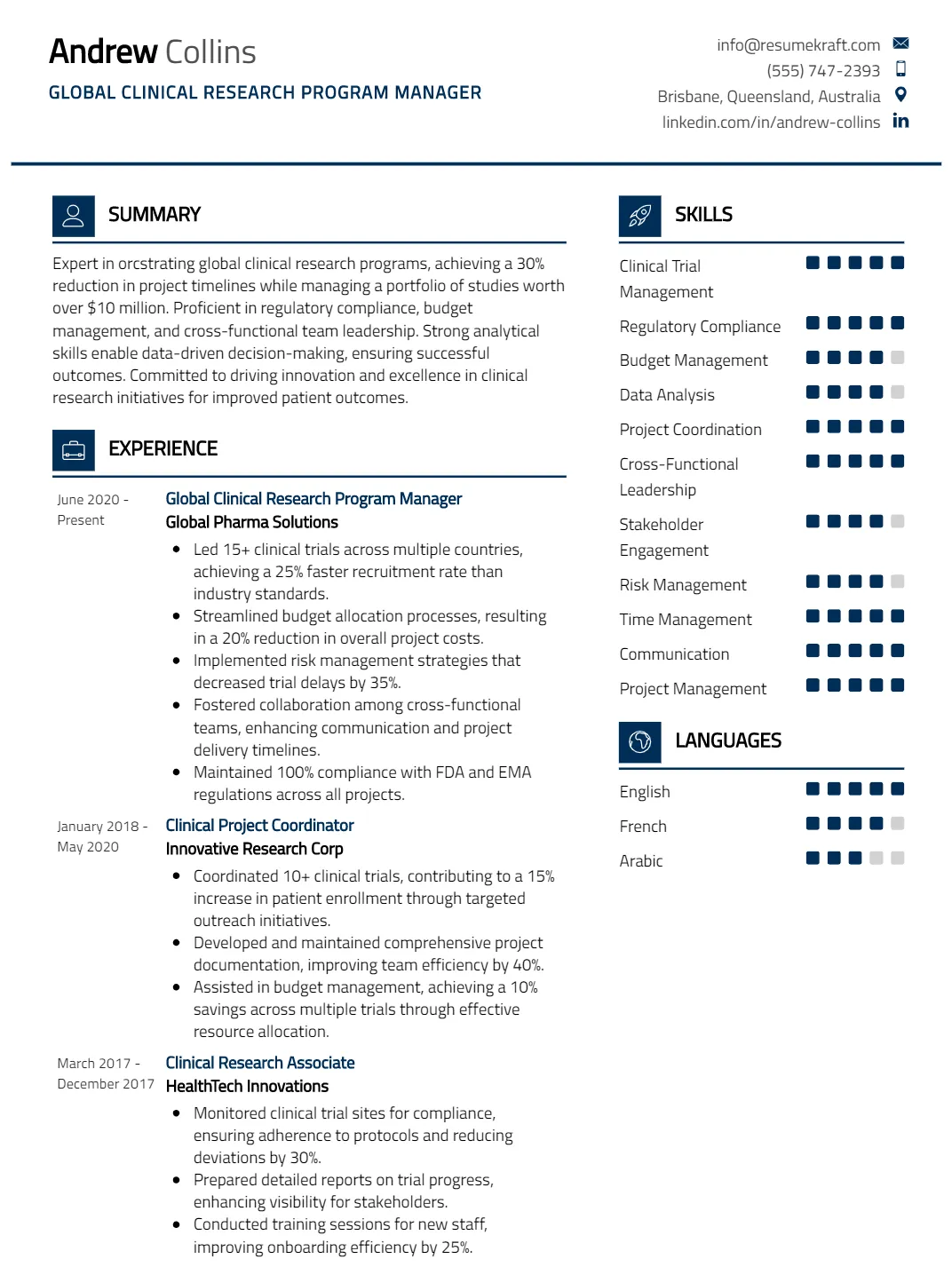
Why This Resume Works
This resume effectively positions the candidate for a Global Clinical Research Program Manager role by highlighting key skills such as Clinical Trial Management and Regulatory Compliance, which are crucial for overseeing complex studies. The structured format allows for easy navigation, showcasing approximately seven years of relevant experience in progressively responsible roles. It incorporates industry-specific keywords to enhance ATS compatibility. Additionally, strategic presentation of achievements demonstrates the candidate’s impact on project outcomes, aligning perfectly with the demands of global clinical research management.
How to format a Clinical Research Coordinator resume
A well-structured resume format is crucial for a Clinical Research Coordinator, as it highlights your relevant skills and experiences, ensuring that hiring managers can easily identify your qualifications and fit for the role.
- Use clear section headings like “Experience,” “Education,” and “Certifications” to guide readers through your qualifications, making it easy to locate pertinent information quickly.
- Keep a consistent font style and size throughout your resume, using bold for headings and standard text for details to maintain a professional and organized appearance.
- Utilize bullet points for listing responsibilities and achievements, as this enhances readability and allows hiring managers to scan your qualifications efficiently.
- Incorporate white space effectively; ensure margins are adequate and that text isn’t cramped, creating a visually appealing layout that encourages thorough reading.
- Prioritize relevant experience at the top of your work history section, focusing on roles and projects related to clinical trials and research to grab attention immediately.
How to write your Clinical Research Coordinator resume experience
Effectively presenting your work experience on a Clinical Research Coordinator resume is crucial, as this section highlights your relevant skills, accomplishments, and the depth of your practical knowledge in clinical trials. Employers seek candidates who can demonstrate their ability to manage complex projects, adhere to regulatory requirements, and collaborate with diverse teams to ensure successful study outcomes.
A well-structured experience section can set you apart from other applicants by showcasing your achievements and quantifying your contributions to previous roles. This not only reflects your expertise but also builds credibility and shows potential employers that you are capable of making a positive impact in their organization.
Worked on clinical trials and did some paperwork. Helped with patient visits and other tasks as needed.
Coordinated 10 clinical trials, managing patient recruitment and regulatory submissions, resulting in a 30% increase in enrollment rates and compliance with all FDA regulations.
How to list your hard skills and soft skills on your resume
A well-crafted resume for a Clinical Research Coordinator should effectively highlight both hard and soft skills to showcase a candidate’s qualifications. Hard skills demonstrate technical competence in research methodologies, data analysis, and regulatory compliance, which are crucial for conducting successful clinical trials. Conversely, soft skills such as communication, teamwork, and problem-solving are equally important, as they enable coordinators to collaborate effectively with diverse teams and navigate the complexities of clinical research environments.
Hard Skills:
- Clinical Trial Management: Proficient in overseeing trial phases from initiation to completion.
- Data Analysis: Skilled in using statistical software to interpret clinical trial data.
- Regulatory Compliance: Knowledgeable about FDA regulations and GCP guidelines.
- Patient Recruitment: Expertise in strategies for enrolling and retaining study participants.
- Informed Consent Process: Experienced in ensuring ethical compliance in participant consent.
- Project Management: Capable of managing timelines, budgets, and resources effectively.
- Medical Terminology: Familiar with the language and terminology used in clinical settings.
- Electronic Data Capture: Proficient in using EDC systems for data collection and management.
- Protocol Development: Skilled in creating and reviewing clinical study protocols.
- Adverse Event Reporting: Experienced in documenting and reporting adverse events accurately.
- Laboratory Procedures: Knowledgeable about clinical lab processes relevant to trials.
- Statistical Analysis: Proficient in applying statistical methods to analyze trial results.
- Quality Assurance: Experienced in maintaining quality standards in research practices.
- Budget Management: Capable of developing and adhering to trial budgets and funding.
- Site Management: Skilled in coordinating activities across multiple clinical trial sites.
Soft Skills:
- Communication: Excellent verbal and written skills for effective stakeholder engagement.
- Teamwork: Ability to collaborate with multidisciplinary teams for successful trial outcomes.
- Problem-Solving: Strong analytical skills to address challenges that arise during studies.
- Time Management: Efficient in prioritizing tasks to meet deadlines in fast-paced environments.
- Adaptability: Flexible in responding to changing project demands and priorities.
- Attention to Detail: Meticulous in maintaining accuracy in data and documentation.
- Empathy: Understanding of participant needs and concerns throughout the research process.
- Leadership: Capable of guiding junior staff and coordinating team efforts.
- Conflict Resolution: Skilled in mediating disputes and fostering a collaborative work atmosphere.
- Interpersonal Skills: Strong ability to build rapport with patients, sponsors, and colleagues.
- Critical Thinking: Proficient in evaluating information and making informed decisions.
- Presentation Skills: Effective in presenting research findings to diverse audiences.
- Negotiation: Ability to negotiate contracts and agreements with vendors and research sites.
- Motivation: Driven to achieve project goals and maintain high standards of research integrity.
- Networking: Skilled in building professional relationships within the clinical research community.
How to list your certifications and education on your resume
When presenting certifications and education on a Clinical Research Coordinator resume, it is essential to prioritize relevance and clarity. List your highest degree first, including the institution’s name, location, and graduation date. Highlight certifications such as Clinical Research Associate (CRA) or Clinical Research Coordinator (CRC) from credible organizations like ACRP or SOCRA, as they showcase your expertise and commitment to the field.
Relevant educational qualifications typically include a degree in life sciences, nursing, or a related field. If you have completed specialized training in clinical research or trial management, ensure it is prominently displayed. This information not only validates your qualifications but also distinguishes you from other candidates.
Graduated from a university. I have some certifications.
Bachelor of Science in Biology, XYZ University, 2021. Certified Clinical Research Coordinator (CCRC) by ACRP, 2022.
How to write your Clinical Research Coordinator resume summary or objective
A strong resume summary or objective for a Clinical Research Coordinator position is crucial as it provides a snapshot of your qualifications and career goals. A summary is best suited for candidates with relevant experience, highlighting key achievements and skills. In contrast, an objective statement is ideal for entry-level applicants or those transitioning careers, focusing on the candidate’s aspirations and what they hope to achieve in the role.
Seeking a position in clinical research. I have some experience and am eager to learn more about coordinating studies and working with participants.
Detail-oriented Clinical Research Coordinator with over 3 years of experience managing clinical trials, ensuring compliance, and improving patient recruitment by 20%. Seeking to leverage expertise in project management and regulatory standards to enhance study outcomes at a leading research facility.
Additional sections for a Clinical Research Coordinator resume
Including additional sections on your Clinical Research Coordinator resume can significantly enhance its impact, showcasing your unique qualifications and experiences. These sections can provide insights into your specialized skills, certifications, and volunteer experiences that make you a strong candidate in the competitive field of clinical research.
- Certifications: Highlight relevant certifications such as Clinical Research Coordinator (CRC) or Good Clinical Practice (GCP). These demonstrate your commitment to professional standards and enhance your credibility in managing clinical trials.
- Relevant Coursework: List specific courses related to clinical research, biostatistics, or ethics. This section showcases your academic foundation and specialized knowledge, reinforcing your qualifications for the role.
- Professional Affiliations: Include memberships in organizations like the Association of Clinical Research Professionals (ACRP). This indicates your dedication to the field and provides networking opportunities that can enhance your career prospects.
- Volunteer Experience: Detail any volunteer work in healthcare or research settings. This reflects your passion for the field and your willingness to contribute beyond paid roles, showcasing your commitment to improving patient outcomes.
- Publications and Presentations: If you have contributed to publications or presented at conferences, include these achievements. This demonstrates your expertise and communication skills, essential for collaborating with teams and sharing research findings.
Key takeaways for writing a professional Clinical Research Coordinator resume
- Highlight your experience with clinical trials, emphasizing your role in patient recruitment, data collection, and regulatory compliance to showcase your expertise in the field.
- Use action verbs and quantifiable achievements to demonstrate your impact, such as the number of studies managed or improvements in patient enrollment rates.
- Tailor your resume to each job application, focusing on relevant skills and experiences that align with the specific requirements outlined in the job posting.
- Consider using resume templates to structure your information clearly and professionally, ensuring that your qualifications stand out to hiring managers.
- Leverage an ai resume builder to streamline your writing process, helping you create a polished and tailored resume that effectively showcases your skills and experiences.
Frequently Asked Questions
How long should my Clinical Research Coordinator resume be?
Your Clinical Research Coordinator resume should ideally be one to two pages long. If you have extensive experience, two pages may be justified to adequately showcase your qualifications and achievements. However, ensure that the content is relevant and focused on your clinical research experience, skills, and accomplishments. Prioritize clarity and conciseness, allowing hiring managers to quickly identify your strengths and suitability for the position.
What is the best format for a Clinical Research Coordinator resume?
The best format for a Clinical Research Coordinator resume is the reverse-chronological format, which highlights your most recent experience first. This format is preferred by employers as it allows them to see your career progression and the relevance of your experience. Additionally, include clear section headings, bullet points for easy readability, and a professional layout. Tailor your resume for each application by emphasizing relevant experience and skills that match the job description.
What should I highlight on my Clinical Research Coordinator resume to stand out?
To stand out as a Clinical Research Coordinator, emphasize your experience in managing clinical trials, knowledge of regulatory compliance, and proficiency in data management. Highlight specific achievements, such as successful trial outcomes, participant recruitment strategies, or protocol development. Additionally, showcase your skills in communication, collaboration with cross-functional teams, and problem-solving abilities. Certifications such as Clinical Research Coordinator (CRC) may also enhance your profile, demonstrating your commitment to the field.
What are some ways to quantify my experience on my Clinical Research Coordinator resume?
Quantifying your experience on your Clinical Research Coordinator resume can significantly enhance its impact. Use metrics to illustrate your achievements, such as the number of clinical trials you managed or the percentage of patient recruitment goals met. Include specifics like the size of budgets managed or the number of participants enrolled in studies. For example, you might state, “Coordinated 10 clinical trials with over 500 participants, achieving a 95% retention rate.” These figures provide concrete evidence of your capabilities.

